A Potentially Treatable Autism-like Brain Disorder Linked to a Problem in Metabolism
By Michael Wheelock, @MSWheelock

Putting the pieces together in brain disease
A young Turkish girl is seated and smiling at a puzzle board in front of her. She places some pieces inside of the board, some ontop of it, some upside down, but none in their correct spot. The girl leans back in her seat and tugs the coat of the clinician next to her, smiling widely. As the movie ends, Dr. Joseph Gleeson pauses briefly to let the images soak in.
Speaking at Rockefeller University this past January, Dr. Gleeson explained that the Turkish girl suffers from an autism-like disorder, a problem in how her brain functions that leads to seizures and impairs her ability to communicate with others. Dr. Gleeson focused on an exciting, recent publication where he identified the potential cause of this girls disorder: a single change in her DNA (a mutation) that puts part of her metabolism into overdrive. Remarkably, mice with the same mutation also suffer from seizures. Even more incredibly, those seizures can be relieved by a simple change in diet!
Man on a mission
Dr. Gleeson is a well-spoken physician who specializes in pediatric brain disease. He is also a neuroscience researcher at UCSD who has dedicated his career to understanding the cause and consequences of brain disease in children. Using a new technology called exome sequencing, Dr. Gleeson has identified potential disease-causing mutations in people with brain disorders.
Searching for the needle in a haystack
Mutations can alter how our cells function, sometimes leading to disease. Researchers like Dr. Gleeson can find these mutations by sequencing (or reading) the DNA of affected patients and comparing it to the DNA of unaffected individuals. The challenge is that any two people have an estimated 3,000,000 differences in their DNA, the overwhelming majority of which are harmless changes (called variations) that have occurred over thousands of years. Dr. Gleeson’s goal is to sort through these variations to find the mutation(s) responsible for a disease (sometimes only a single change). Talk about a needle in a haystack!
How does Dr. Gleeson do this? One approach would be to read the ~3 billion letters of DNA in a patient (called their genome) to find the mutation(s). However, this process is expensive (among other things) and most of the letters that get read are unrelated to the actual disease. So rather than sequence all of a patient’s DNA, Dr Gleeson focuses on the ~1% of DNA that makes proteins, called the exome. Proteins are like tools the cell uses to carry out its functions. The exome is like a set of blueprints for how each tool is assembled. If there is a change in the blueprint (a mutation in the exome), the tool may no longer function properly. This can alter the cell’s function, leading to disease. Thus, the exome is a great place to look for disease-causing mutations.
Dr. Gleeson focuses on inheritable forms of brain disease (those caused by mutations passed on to a child by his/her parents). More specifically, Dr. Gleeson looks for recessive mutations, a type of mutation that causes disease only if inherited from both the mother and the father (this gives the child two mutant copies and no regular copies). The reason? If the mutation is recessive, you know an affected child should have two copies and their parents should each have one copy (because they are unaffected, but have given the mutation to their child). This prediction makes finding the disease-causing mutation much easier
Linking brain disease to metabolism
Here is where the young Turkish girl comes in. She was diagnosed by one of the physicians Dr. Gleeson has partnered with in northern Africa and central Asia to help find mutations associated with brain disease. After comparing this girl’s exome to those of her immediate family, Dr. Gleeson found a single mutation that correlated with her disease.
The mutation was in the blueprint for a protein called BCKDK. This protein controls how the body uses a group of amino acids called BCAAs. Amino acids generally have two uses: they can be used to build the many proteins in a cell or they can be converted into other useful products. Since we only obtain BCAAs from our diet, our bodies must control how they are used to ensure a sufficient amount of BCAAs and the products they’re converted to (this control is part of our metabolism).
Normally, BCKDK acts as a brake to prevent the conversion of BCAAs. However, the mutation in the young Turkish girl removed a part of the protein required for this function. Dr. Gleeson predicted that removing this brake would cause the Turkish girl to convert too many of her BCAAs to other products, leaving behind fewer intact BCAAs. Indeed, the girl had very low levels of BCAAs in her blood compared to her unaffected family members (Figure 1).
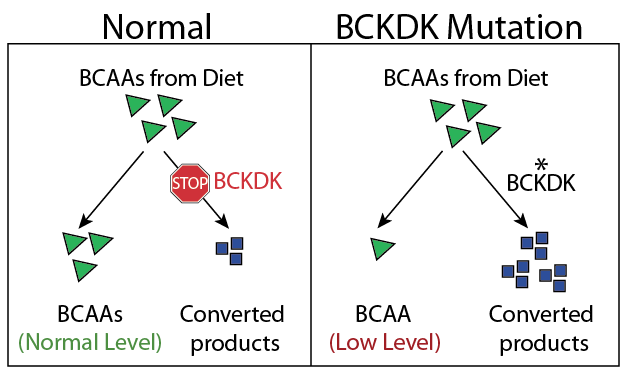
To investigate the effect of low amounts of BCAAs in the blood, Dr. Gleeson raised mice with the same mutation in BCKDK as the Turkish girl (mice are much easier to study than humans!). Similar to the girl, adult mice with two copies of the mutation suffered from seizures and had low levels of BCAAs in their blood. Dr. Gleeson then analyzed the amino acids in the brain of affected mice and found something intriguing: in addition to low levels of BCAAs, these mice had higher levels of a more general category of amino acids called LNAAs. These amino acids are used to make neurotransmitters which are chemicals used by brain cells to communicate with each other. This increase in LNAAs was specific to the brain (levels in the blood were fine), pointing towards a possible cause for the brain disorder.
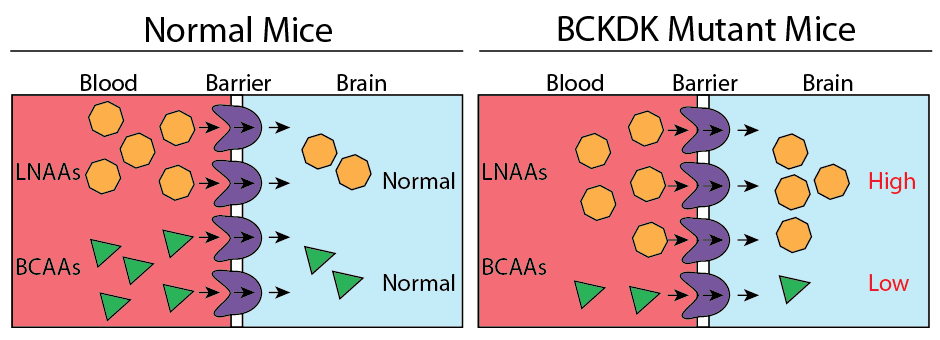
Next, Dr. Gleeson was interested in how the decrease in BCAAs in the blood could increase the amount of LNAAs in the brain. Just like other organs, the brain requires amino acids to function properly. While most of our body obtains amino acids from the blood, the brain is a special case. A barrier (aptly named the blood-brain barrier) prevents blood from coming into direct contact with the brain (this helps keep it safe from infection, among other things).
This barrier has gates called transporters that can move certain amino acids from the blood into the brain. Since these transporters can only move one amino acid at a time, amino acids compete with each other for passage through the gate. If mutating BCKDK leads to low levels of BCAAs in the blood, the gate will keep working, except now there are only LNAAs to move, elevating their level in the brain (Figure 2). Since LNAAs are used to make neurotransmitters, this elevation might change how neurons communicate with each other, eventually causing problems in brain function.
Finally, Dr. Gleeson was curious if he could restore the balance of amino acids in the blood and brain of the mutant mice by adding extra BCAAs to their diet. This would help compensate for how quickly the mice metabolize them. Amazingly, this treatment not only restored amino acid levels, but also completely stopped the mice’s seizures (Figure 3)! Switching these mice back to a normal diet caused the seizures to return as the mice quickly metabolized their BCAAs.
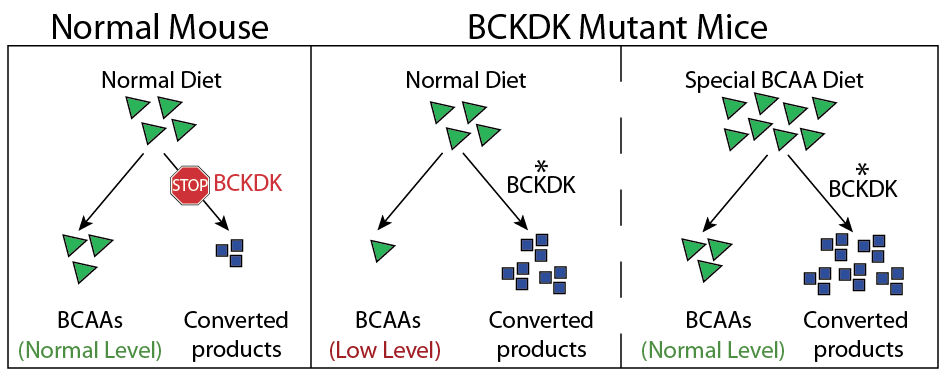
This work suggests that modifying the diet of patients with a BCKDK mutation might treat some of their symptoms. It is important to remember that these results are limited to mice and require further investigation. However, Dr. Gleeson is hopeful that ongoing work in his lab, including testing the effect of dietary modification on the Turkish girl and other patients with BCKDK mutations, may lead to a treatment for this particular Autism-like disorder.
This has been brought to you by:

Michael Wheelock is a birder, basketball player, and graduate student at The Rockefeller University. Catch him on twitter @MSWheelock and follow his series #TheBirdphiles.

1 thought on “A Potentially Treatable Autism-like Brain Disorder Linked to a Problem in Metabolism”